970x125
The ability to extract and analyse genomic DNA from ancient human remains has revolutionised our understanding of human evolution. The genome is the full set of genetic instructions within an organism, containing all the information necessary for its development, function, and survival. It serves as a detailed blueprint or instruction manual, guiding the formation and operation of life—from the simplest organisms to the most complex. Genome is also the substrate for evolution, and variations in the genome reflect the evolutionary history of the organisms. The field of genomics studies genomes—how they function, evolve, and influence health and disease.
970x125
One of the most transformative discoveries in this field is the evidence that modern humans interbred with now- extinct hominid species, such as Neanderthals and Denisovans. Genomic studies have revealed that after early modern humans migrated out of Africa, they encountered and mated with these archaic humans. As a result of this interbreeding, individuals of European and Asian descent today carry a small but measurable percentage of Neanderthal DNA—typically around 1–2%. Populations in Southeast Asia and Melanesia show even higher proportions of Denisovan DNA. Such findings refine the geographical and timeline narrative of the evolution of modern humans and the populating of the planet by people.
These remnants of ancient genomes are not just evolutionary curiosities; they continue to influence our biology today. Comparative genomics of ancient and modern human DNA, in addition to providing profound insights into our evolutionary history, uncover biological adaptation and disease susceptibility. Some Neanderthal-derived genes are known to affect immune system function, making certain populations more resistant—or in some cases, more susceptible—to specific diseases including COVID-19. Meanwhile, Denisovan DNA has been linked to traits like high-altitude adaptation in Tibetan populations, enabling them to thrive in low-oxygen environments.
Altogether, these discoveries have fundamentally changed our view of human origins. Rather than a linear or isolated evolution, they depict a dynamic and intertwined history marked by migration, interaction, and genetic exchange across different human species. This complex evolutionary tapestry underscores the shared and diverse heritage of humanity.
Charting India’s genetic landscape
India, with over 1.4 billion people and nearly 5,000 distinct communities, is one of the world’s most genetically diverse regions. Yet, it is underrepresented in major global genomic studies. While projects like the 1000 Genomes Project, UK Biobank, and others have included some Indian samples, most focused on expatriate populations and offered limited insight. As a result, major questions about the origins, migrations, and disease risks in Indian populations remain unresolved.
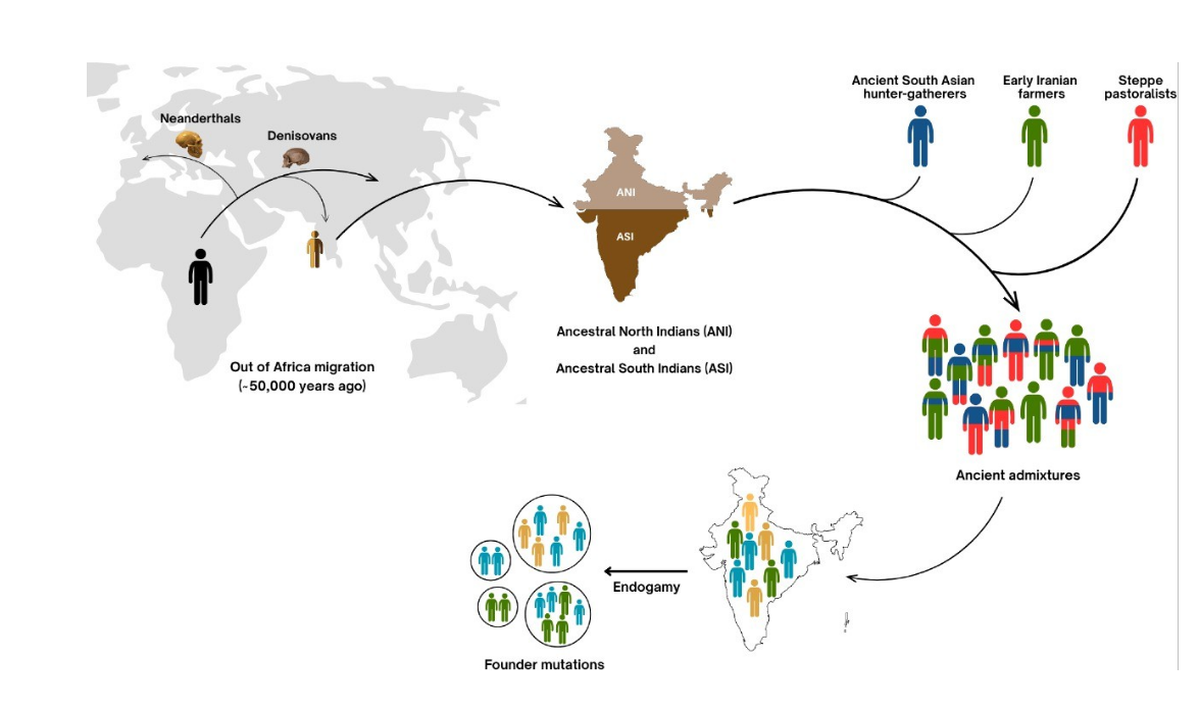
India’s genetic heritage stems from a single out-of-Africa migration roughly 50,000 years ago, followed by interbreeding with Neanderthals and Denisovans. Early mixing between Ancestral North Indians (ANI) and Ancestral South Indians (ASI) produced a distinct genetic signature. Subsequent contributions from Ancient South Asian hunter-gatherers, Early Iranian farmers, and Steppe pastoralists further enriched this diversity. While ancient admixture shaped the deep genetic framework, more recent practices of endogamy have preserved this variation within ~5,000 distinct ethnic groups (as represented in different coloured dots), essentially locking in the genetic landscape of earlier eras. This has led to the fixation and enrichment of founder mutations, offering valuable insights into gene function and disease biology. Illustration: Courtesy, Meghana Muskan
To address this, researchers conducted one of most comprehensive genetic study of Indian populations to date, including people from diverse regions, language families, and underrepresented tribal groups (Lalji Singh and Thangaraj group, Nature Genetics, 2017). The study revealed that most genetic variation in Indians comes from a single major human migration out of Africa around 50,000 years ago. These early settlers later interbred with archaic human species such as Neanderthals (Europe and west Asia) and Denisovans (central, eastern, and southeastern Asia). This study highlighted the complex demographic history of India, revealing significant intermixing between two ancestral groups: the Ancestral North Indians (ANI) and Ancestral South Indians (ASI). This early admixture shaped the unique genetic landscape seen today, reflecting ancient migrations and population interactions over millennia. The research demonstrated that Indian populations are genetically distinct from other global groups, underlining the country’s rich genetic diversity. Importantly, this work laid the foundation for understanding genetic predispositions to diseases prevalent in India, informing personalized medicine and public health strategies. By integrating geographical and temporal contexts, the study also contributed to reconstructing India’s population history. Overall, Singh’s work is a milestone in human genetics, emphasising the need to study indigenous populations to unlock health and evolutionary insights unique to India’s genetic heritage.
Another landmark genomics study published recently ((Moorjani and coworkers, Cell, June 2025) has revealed that most Indians have ancestry from three ancient groups: Ancient South Asian hunter-gatherers (AHG), Early Iranian farmers, and Steppe pastoralists. DNA from ancient Iranian populations, particularly from 3600–3500 BCE in present-day Tajikistan (Sarazm), shows that early farmers and herders contributed to the ancestry of various Indian populations, including Ancestral North and South Indians, Austroasiatic speakers, and East Asian-related groups. Archaeological findings also confirm trade and cultural connections with early South Asian civilizations like Mehrgarh and the Indus Valley.
The study also explored the contribution of Neanderthal and Denisovan ancestry. Indians carry 1–2% of their DNA from these archaic humans and show the highest diversity in archaic DNA among all modern populations. Notably, some of these ancient variants affect immune function. For example, Neanderthal genes on chromosome 3 are associated with severe COVID-19 symptoms, while Denisovan variants are found in immune-related genes like those in the MHC and TRIM families. Additionally, researchers found “ancestry deserts”—genomic regions that lack Neanderthal or Denisovan DNA. One such region includes the FOXP2 gene, linked to language development in humans.
Taken together, these studies highlight India’s deep and complex genetic history shaped by ancient migrations, endogamy, and archaic gene flow. Understanding this diversity is essential for developing accurate, inclusive, and effective medical and genomic research tailored to Indian populations.

Endogamy and genetic diseases
Over time, many Indian communities adopted endogamy—marrying within their group—leading to a high degree of genetic relatedness. The 2017 Nature Genetics study co-led by Kumarasamy Thangaraj analysed genome data from over 2,800 individuals across 275+ South Asian populations, revealing strong founder effects and endogamy (marriage within groups) in about one-third of these groups. This genetic isolation causes a high prevalence of population-specific rare recessive diseases unlike those seen elsewhere. For example, the Vysya community has a 100-fold higher rate of butyrylcholinesterase enzyme deficiency due to such founder events. The study demonstrated population-specific recessive disease gene mapping, such as identifying a mutation causing Progressive Pseudorheumatoid Dysplasia in southern Indian patients mostly from non-consanguineous marriages.
The 2025 study led by multiple groups using genomes of about 2,700 individuals reported that on average, each person in the study had at least one fourth-degree relative, and levels of homozygosity (inheriting identical copies of genes) were significantly higher than in East Asian or European populations. While endogamy strengthened community ties, it also increased the risk of genetic disorders due to recessive disease-causing variants. Researchers identified more than 1,60,000 previously unreported genetic variants, many linked to congenital conditions, blood disorders, metabolism, drug responses, and diseases such as dementia. These variants are unique to Indian populations—rare across the country but often common in specific groups.
These studies mark a shift in Indian genetic research from focusing on common diseases to exploring rare, population-specific disorders, paving the way for personalised medicine. They emphasise the importance of community-based genetic screening and counseling to ease the disease burden. By drawing on the distinct population history of South Asia’s thousands of endogamous groups, this approach enables earlier diagnosis and more precise interventions.
Also Read:Decoding the script: On the Genome India Project and its sequencing 10,000 Indian genomes
India’s genetic mosaic: a frontier for personalised medicine
India is home to unparalleled human genetic diversity, comprising more than 5,000 distinct ethnic groups, each shaped by unique social, geographical, and cultural practices. Long-standing traditions of endogamy across these groups have resulted in the accumulation of numerous founder mutations, making the Indian population one of the richest repositories of naturally occurring genetic variants anywhere in the world. This diversity presents a transformative opportunity for India – not only to deepen scientific understanding of human gene function and biology but also to revolutionise the delivery of healthcare through precision and personalised medicine. The natural presence of variants across populations enables us to ask biological questions that cannot be replicated in model systems or generated through laboratory-induced mutations. With appropriate infrastructure and vision, India can position itself as a global leader in genome-driven science.

Future perspectives
India possesses a tremendous opportunity to leverage its population’s genetic diversity. The successful sequencing of 10,000 Indian genomes (the Genome India project of Department of Biotechnology, Government of India) is a significant milestone, providing a valuable foundation for future research. This wealth of genomic data will be crucial for attracting stakeholders, including the pharmaceutical and biotechnology industries.
Building on this success, the next phase should involve large-scale genome sequencing including millions of the Indian population in the context of various diseases and clinical features. We should also prioritise the creation of a national biobank modeled on the UK Biobank with longitudinal follow-up. This will be pivotal for advancing precision healthcare and driving a genomics industry revolution in the country. By pursuing this, India can not only improve the health of its population but also establish itself as a global leader in the field of genomics and genomics based medicine.
(Dr. Rakesh Mishra is director, Tata Institute for Genetics and Society, Bangalore. rakesh.mishra@tigs.res.in; Prof Sanjeev Galande is Team Leader, Center of Excellence in Epigenetics Dean, School of Natural Sciences, Shiv Nadar Institute of Eminence. sanjeev.galande@snu.edu.in)
970x125

